We have witnessed technology adoption in nearly every industry through the digitization of manual tasks. Pathology laboratories are no exception, and we are also observing a transition to digital workflows in this field. A digital pathology workflow involves the scanning of glass slides into Whole-Slide Images (WSI), which are subsequently analyzed and digitally stored.
The digitization of laboratories has the potential to alleviate the challenges associated with analog processes. However, like any significant shift, this transformation also presents its own barriers. To ensure a seamless transition, it’s crucial to understand how to effectively address these challenges. In this blog, we offer an overview of the advantages of digitalization, expected challenges, and strategies for mitigating those challenges when adopting a digital workflow in pathology laboratories.
The trends driving digital adoption in pathology
Digital adoption is also taking place in the field of pathology, primarily driven by the following key factors:
The decline of available pathologists combined with the rising number of cancer cases.
The number of retiring pathologists exceeds the number of new pathologists entering the field. The latter, coupled with an increase in cancer cases due to an aging population, is placing a heavier workload on pathologists1. This increasing demand requires solutions that enhance workflow efficiency and quality.
The emergence of AI tools designed for pathology.
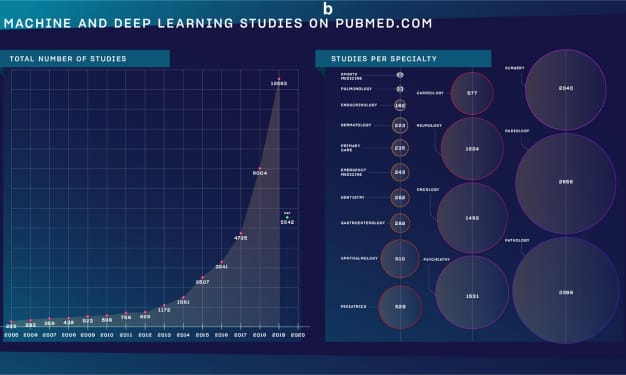
Due to the escalation of workload on pathologists, the need for AI tools has been recognized by many tech companies, resulting in a significant increase in AI publications in the field of pathology2. These tools offer assistance in identifying rare events, standardizing the tumor diagnosis and grading process, introducing greater quantification into histopathological analyses, and aiding in various other tasks. However, the implementation of these solutions requires first a transition to a digital workflow.
The increasing need to work remotely.
During the COVID-19 pandemic, pathology laboratories in the process of implementing a digital workflow accelerated the transition, enabling pathologists to work remotely from their homes.2 Today, the pandemic is over, but a significant number of professionals continue to favor hybrid working modes, drawn by the advantages they offer.
What are the benefits of digital pathology?
Implementation of digital pathology can have significant improvements on the laboratory workflow, some of them being:
Remote collaboration.
Digital pathology enables remote collaboration as images are stored on a server and accessible through web-based platforms from anywhere at any time.
AI-powered image analysis.
Digital images can be analyzed by AI software for multiple tasks, automating pre-analytical processes, and providing data-driven insights that improve clinical decision-making. This offers the potential for more efficient, accurate, and standardized results.
Reduced time retrieving digital slides.
Digital data storage facilitates convenient access to archived digital slides, maintaining their quality for the long term. These images are easily searchable, and depending on the storage type, they may be accessible from multiple locations.
Enhanced Viewing.
Whole slide images (WSIs) can then be viewed using desktop viewers, enabling pathologists to examine the entire specimen slide on-screen with the ability to zoom and view from different angles.
What challenges can we expect?
The introduction of digital pathology brings forth several challenges, including:
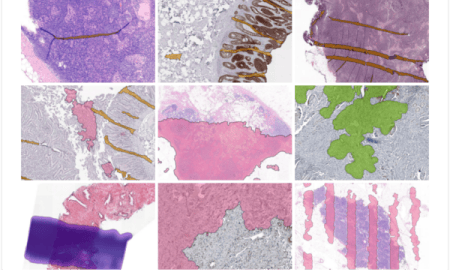
Presence of image artifacts.
It’s crucial to perform quality control immediately after the scanning process to identify artifacts introduced during the preparation or scanning of slides. The presence of these artifacts (e.g. out-of-focus areas) can hinder the examination of tissue sections or delay the diagnosis. Efficient quality control ensures timely decisions regarding rescanning slides, cleaning the glass, or producing new slides. Failure to address issues during this phase may lead to pathologists detecting problems during the diagnostic review, necessitating communication back to earlier workflow stages and causing delays.
Digitization of archived glass slides.
Some laboratories transitioning to digital pathology also wish to digitize their archive of glass slides. However, the prevalence of quality artifacts within these samples tends to be high. Factors such as dust accumulation or the presence of pen markings contribute to these artifacts, potentially impeding an efficient transition to a fully digital system.
Storage space.
Whole Slide Images can consume a significant amount of storage space. This is particularly problematic for laboratories with long retention policies and those that are required to archive the slides on-premise. An alternative is storing images in the cloud, however, this method can become expensive. Another alternative is archival storage. Nonetheless, it comes with the drawback of the sample not being immediately accessible.
Remote working limitations.
While digital pathology provides pathologists the flexibility to work remotely, challenges may emerge, particularly in rural areas where slow internet connections are prevalent. There are also variations between office and personal display setups, presenting additional obstacles. For instance, the smaller dimensions of personal computers may not accommodate certain graphic cards, potentially causing delays in case handling. Additionally, the absence of remote access to all data and tools can be a significant issue3.
Challenge mitigation solutions
Automated quality control step.
Implementing an automated quality control (QC) AI solution in a digital workflow can reduce the workload caused by manual inspection of artifacts. By having a QC algorithm that flags artifacts before the lab technician or pathologist begins their assessment, technicians can efficiently reject flagged slides or send them for further review, saving valuable time. This helps diagnostic and research laboratories to ensure that only high-quality WSIs are forwarded for review and prevents the unnecessary upstream referral of cases by pathologists.
Storage strategy.
Tailoring a sustainable and cost-efficient storage strategy in digital pathology hinges on factors like daily slide scans, retention policies, and storage purpose. The advantage in pathology lies in the fact that once the diagnosis is complete and reported, the image is unlikely to be needed again. This enables the use of archival storage, a cheaper and sustainable alternative to cloud storage, though harder to retrieve. It is crucial for all stakeholder groups to collaboratively decide which slides are important to have immediately available and which ones are essential for long-term retention but are unlikely to be revisited. However, on-premise storage constraints in hospitals may require physical slide storage with rescanning upon revisitation4.
Proper IT setup.
To address the challenges associated with pathologists working from home, it is helpful to establish standardized IT setups to prevent variations that may impact workflow efficiency. Additionally, providing secure remote access to all necessary data and tools is crucial, and technologies like a secure web image viewer can facilitate this. Overall, a fitting IT infrastructure requires active involvement and proactive measures from the IT team during the transition to digital3.
Even though digital pathology is a convenient alternative to the traditional lab, the transition is not without its challenges. By effectively addressing them, pathology laboratories can avoid unnecessary struggles and fully experience the benefits of a digital workflow.
Sources
- Robboy, S. J., Weintraub, S., Horvath, A. E., Jensen, B. W., Alexander, C. B., Fody, E. P., Crawford, J. M., Clark, J. R., Cantor-Weinberg, J., Joshi, M. G., Cohen, M. B., Prystowsky, M. B., Bean, S. M., Gupta, S., Powell, S. Z., Speights, V. O., Gross, D. J., & Black-Schaffer, W. S. (2013). Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Archives of Pathology & Laboratory Medicine, 137(12), 1723–1732. https://doi.org/10.5858/arpa.2013-0200-oa
- Meskó, B., & Görög, M. (2020). A short guide for medical professionals in the era of Artificial Intelligence. Npj Digital Medicine, 3(1). https://doi.org/10.1038/s41746-020-00333-z
- Schwen, L. O., Kiehl, T.-R., Carvalho, R., Zerbe, N., & Homeyer, A. (2023). Digitization of Pathology Labs: A Review of Lessons Learned. Laboratory Investigation, 103(11), 100244. https://doi.org/10.1016/j.labinv.2023.100244
- Scarisbrick, C. (2022, September 12). Has the cloud industry solved a big problem for digital pathology? Sectra Medical. https://medical.sectra.com/resources/has-the-cloud-industry-solved-a-big-problem-for-digital-pathology/
More Blogs
-
AI assistance in Chronic Kidney Disease monitoring
03 July, 2024 • By Kama Witkowska
Read more -
Behind the scenes of pathology AI innovation – Blazej Dolicki’s insights into training, validation, deployment, and beyond
18 June, 2024 • By Victoria Grosu
Read more -
A short overview of the history of pathology: origins, early days, and the transition to novel technologies
16 May, 2024 • By Kama Witkowska
Read more -
The benefits of AI implementation in mitotic figure counting
11 April, 2024 • By Kama Witkowska
Read more -
Diana Rosentul explains the complexities of regulatory compliance for integrating AI-based solutions in the healthcare industry
25 January, 2024 • By Diana Rosentul
Read more -
Cristina González Gonzalo discusses her journey with the clinical validation of Aiosyn Mitosis Breast
14 December, 2023 • By Anna Correas Grifoll
Read more -
Prepare for the future: AI is changing the landscape of pathology
09 October, 2023 • By Victoria Grosu
Read more -
How automated quality control of whole slide images can increase the efficiency of clinical and research pathology workflows
25 July, 2023 • By Victoria Grosu
Read more