Cristina González Gonzalo, Clinical Program Manager at Aiosyn, shares her experiences from the clinical validation of Aiosyn Mitosis Breast. Joining the team in May 2022, she played a crucial role in validating Aiosyn’s mitosis detection AI algorithm. In this interview, she provides insights into ensuring the study’s results are representative, discusses the challenges faced, and outlines her approach to coordinating collaboration with Radboud University Medical Center.
“I am highly satisfied with the results of the study, since the clinical benefits of Aiosyn Mitosis Breast we wanted to validate have been confirmed: pathologists do become more efficient and consistent when they are assisted by our device, without compromising their accuracy.”
Cristina González Gonzalo, Clinical Program Manager
Anna Correas, Marketing & Communications Specialist at Aiosyn: Good morning, Cristina. First of all, congratulations on the first results of the clinical performance study. It’s great to see how AI can benefit pathologists in a clinical setting. Before diving into the study, could you please share a bit about your background?
Cristina González Gonzalo, Clinical Program Manager: Certainly! I have a background in biomedical engineering and computer vision, with a PhD on AI in medical image analysis (Radboud University Medical Center / University of Amsterdam). My background helps build a bridge between the AI and clinical worlds, which turned out to be pretty crucial for the clinical study. Understanding the needs and requirements from both sides was key to making the study work.
Anna: What was the objective of the clinical study?
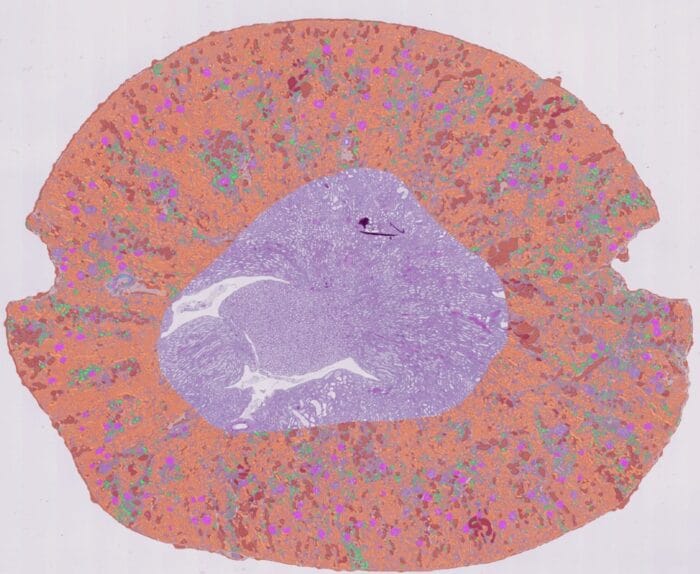
Cristina: The main objective of the study was to validate, within the framework of the In Vitro Diagnostic Regulation (IVDR), the clinical benefits of Aiosyn Mitosis Breast for mitosis counting as part of breast cancer diagnostics. For this purpose, the device had to be used by intended users (pathologists) in an intended use environment (in this case, using a CE-certified digital pathology viewer). We wanted to study the potential of Aiosyn Mitosis Breast to increase the efficiency and consistency of pathologists, while maintaining their accuracy, in comparison to current clinical practice (i.e., unassisted, manual mitosis counting).
Anna: What have been your main responsibilities during the clinical study?
Cristina: As Clinical Program Manager, my main responsibilities spanned across the different phases of the study. In the initial stages, I led the design of the study’s methodologies and the required resources, overseeing the arrangement of samples and participants, as well as the configuration of the device and the study environment. Throughout the study, I supervised the adherence to the established plan and timelines, as well as to regulatory requirements. I played a key role in coordinating efforts between the Radboud university medical center and Aiosyn teams, overseeing data collection and participant engagement. As we approached the end of the study, I took charge of supervising the analysis of all collected data and ensured timely reporting in compliance with applicable standards and regulations.
Anna: What important factors did you consider when organizing the study to ensure the results obtained are representative of what we can expect in a real clinical setting?
Cristina: First, working on a meticulous plan and design of the study, engaging pathology experts. Their insights were invaluable in shaping methods and configuring the study environment to extract results that are not only valid but also representative. Second, throughout the sample collection and participant recruitment phase, we prioritized diversity. This included ensuring representation across centers and subgroups of the most relevant clinical variables among samples, and varying levels of experience in breast pathology and digital pathology among participants, thereby mitigating potential biases. Additionally, a critical aspect was the implementation of a thorough statistical analysis of the collected data, aimed at producing results that are verifiable and reproducible, contributing to the study’s reliability and applicability to real clinical settings.
Anna: What challenges did you encounter?
Cristina: I would say that coordinating a large group of participants (28 readers and 3 pathologists subspecialized in breast pathology) came with its logistical challenges. The coordination between multiple teams at Radboud university medical center and Aiosyn brought an additional layer of complexity, demanding effective communication strategies. In addition, a seamless collection of samples across multiple centers was a priority, and this required constant vigilance to adhere to ethical guidelines in data transfers and management. On top of these challenges were tight deadlines, underscoring the need for efficient time management and decision-making to ensure the study’s success.
Anna: How was coordinating this study with Radboud university medical center?
Cristina: Coordinating the study with Radboud university medical center was a very positive experience. While it was the first time both the team at Radboud university medical center and Aiosyn were running a clinical study under within the scope of IVDR, their extensive prior experience with clinical studies truly helped in navigating the complexities. The collaboration benefited significantly from Radboud’s state-of-the-art facilities and the expertise of their team (Jeroen van der Laak, Leslie Tessier, Maschenka Balkenhol). They have been key in the study’s success, undoubtedly.
Anna: Are you satisfied with the results of the clinical performance study? Were there any surprises?
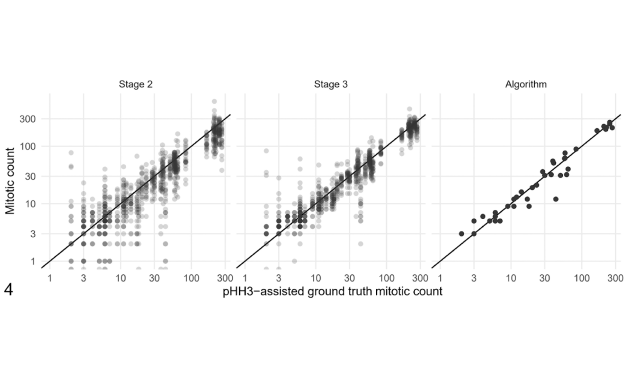
Cristina: I am highly satisfied with the results of the study, since the clinical benefits of Aiosyn Mitosis Breast we wanted to validate have been confirmed: pathologists do become more efficient and consistent when they are assisted by our device, without compromising their accuracy. As for surprises, the study reaffirmed the high levels of inter-observer variability among pathologists in mitosis counting. While not entirely unexpected, this finding underscores the potential of AI support in standardizing care.
Anna: When will the study be published?
Cristina: A peer-reviewed publication of the study is currently in progress, and we anticipate its release next year.
Anna: When do you think we’ll start to see some clinics using Aiosyn Mitosis Breast?
Cristina: The initial step is obtaining CE certification for the device, and we view the results of this study as a very valuable piece of evidence for the certification process. Given the current interest we are receiving from pathologists across various centers for integrating Aiosyn Mitosis Breast into their workflows, I anticipate a swift adoption once the device is certified.
Anna: Thank you, Cristina, for your time! I look forward to reading the study’s publication once it is out and to witnessing the adoption of Aiosyn Mitosis Breast in clinical workflows.
Cristina: Thank you for the interview. I am also excited about seeing the positive impact of AI-powered mitosis detection on doctors and patients.
To be among the first to read the study’s publication and stay updated on Aiosyn news, consider subscribing to our newsletter or following us on LinkedIn.

More Blogs
-
AI assistance in Chronic Kidney Disease monitoring
03 July, 2024 • By Kama Witkowska
Read more -
Behind the scenes of pathology AI innovation – Blazej Dolicki’s insights into training, validation, deployment, and beyond
18 June, 2024 • By Victoria Grosu
Read more -
A short overview of the history of pathology: origins, early days, and the transition to novel technologies
16 May, 2024 • By Kama Witkowska
Read more -
The benefits of AI implementation in mitotic figure counting
11 April, 2024 • By Kama Witkowska
Read more -
Diana Rosentul explains the complexities of regulatory compliance for integrating AI-based solutions in the healthcare industry
25 January, 2024 • By Diana Rosentul
Read more -
Time to go digital? Navigating the pathology transition from microscopes to whole slide images
30 November, 2023 • By Victoria Grosu
Read more -
Prepare for the future: AI is changing the landscape of pathology
09 October, 2023 • By Victoria Grosu
Read more -
How automated quality control of whole slide images can increase the efficiency of clinical and research pathology workflows
25 July, 2023 • By Victoria Grosu
Read more