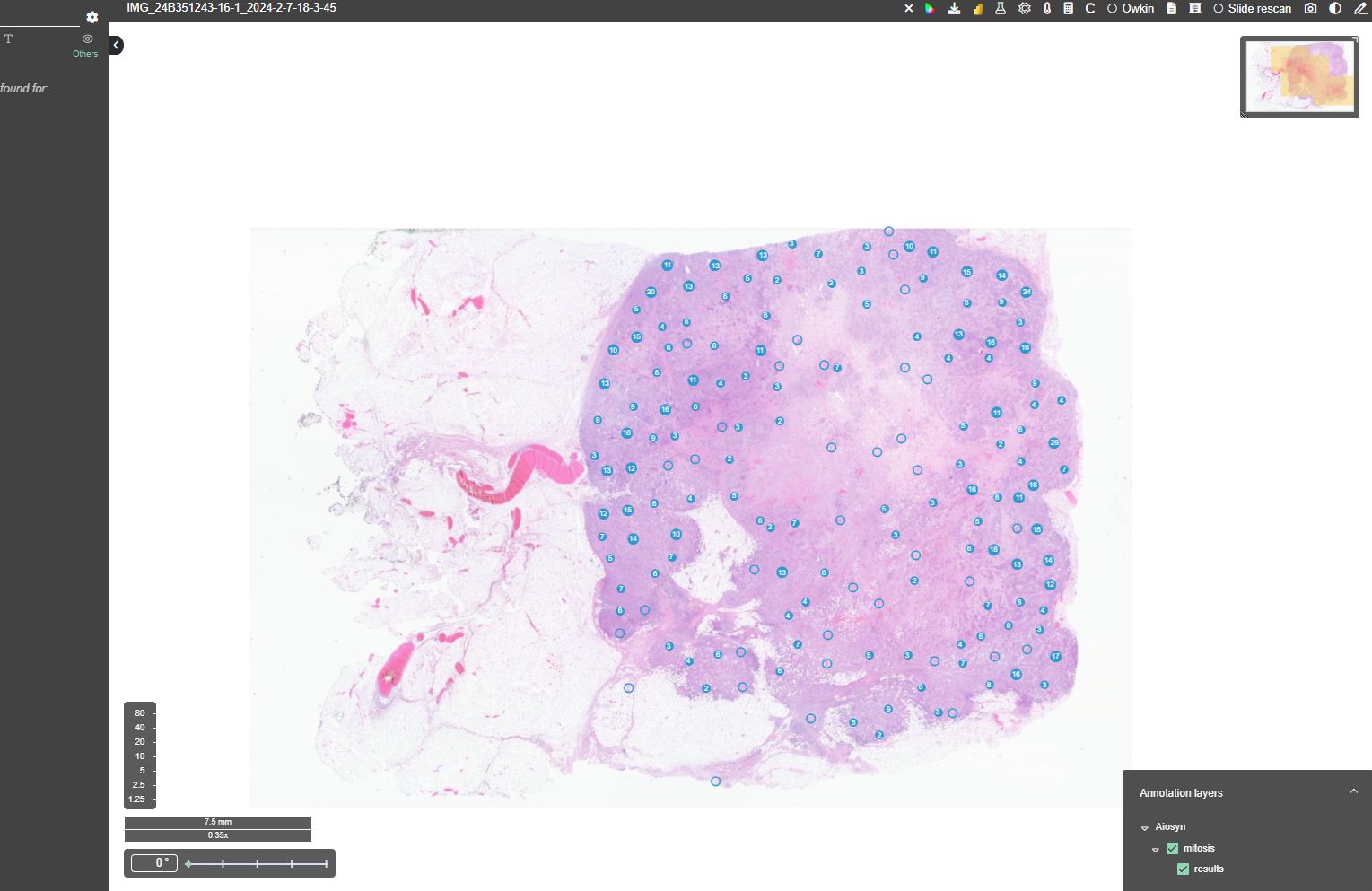
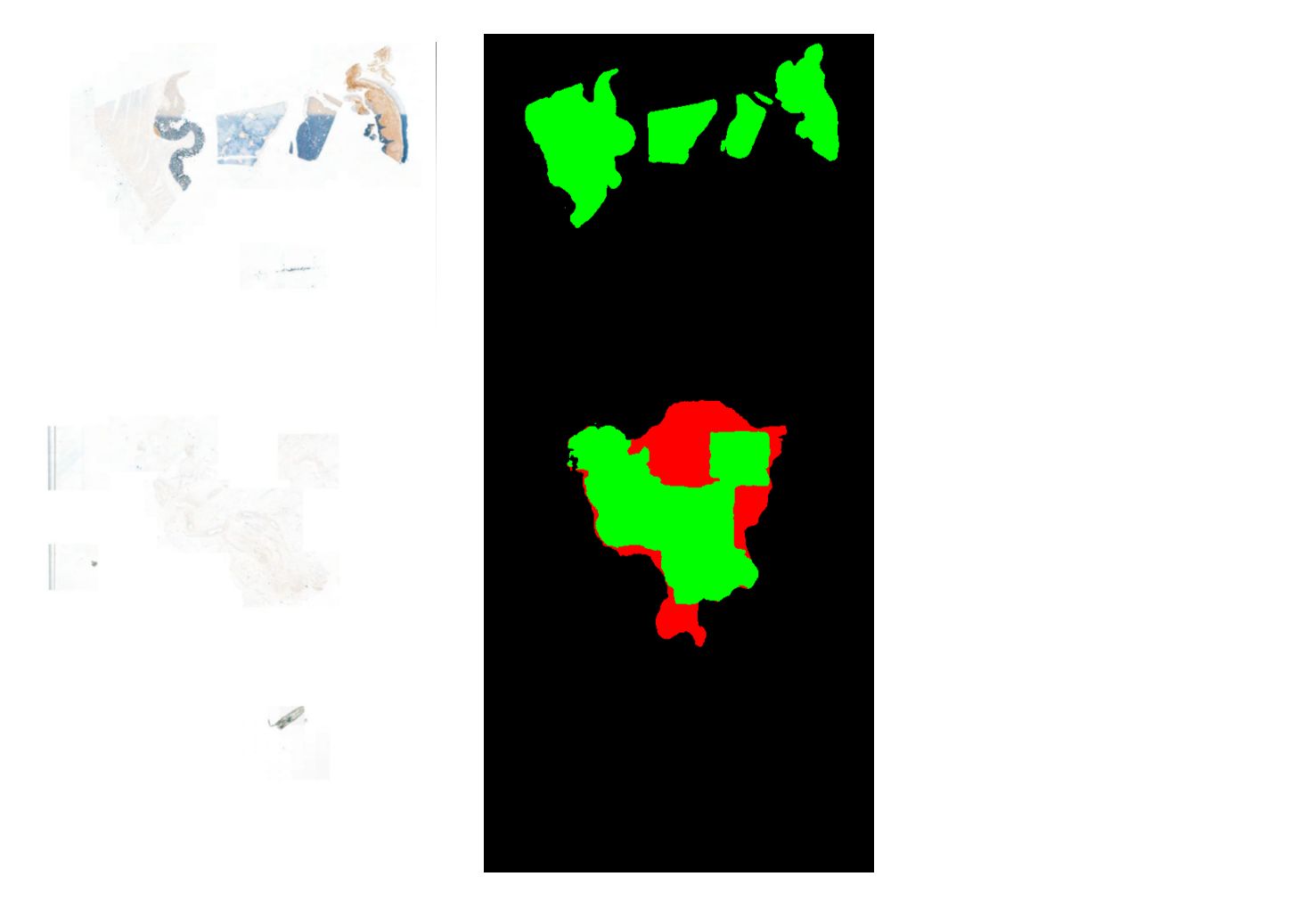
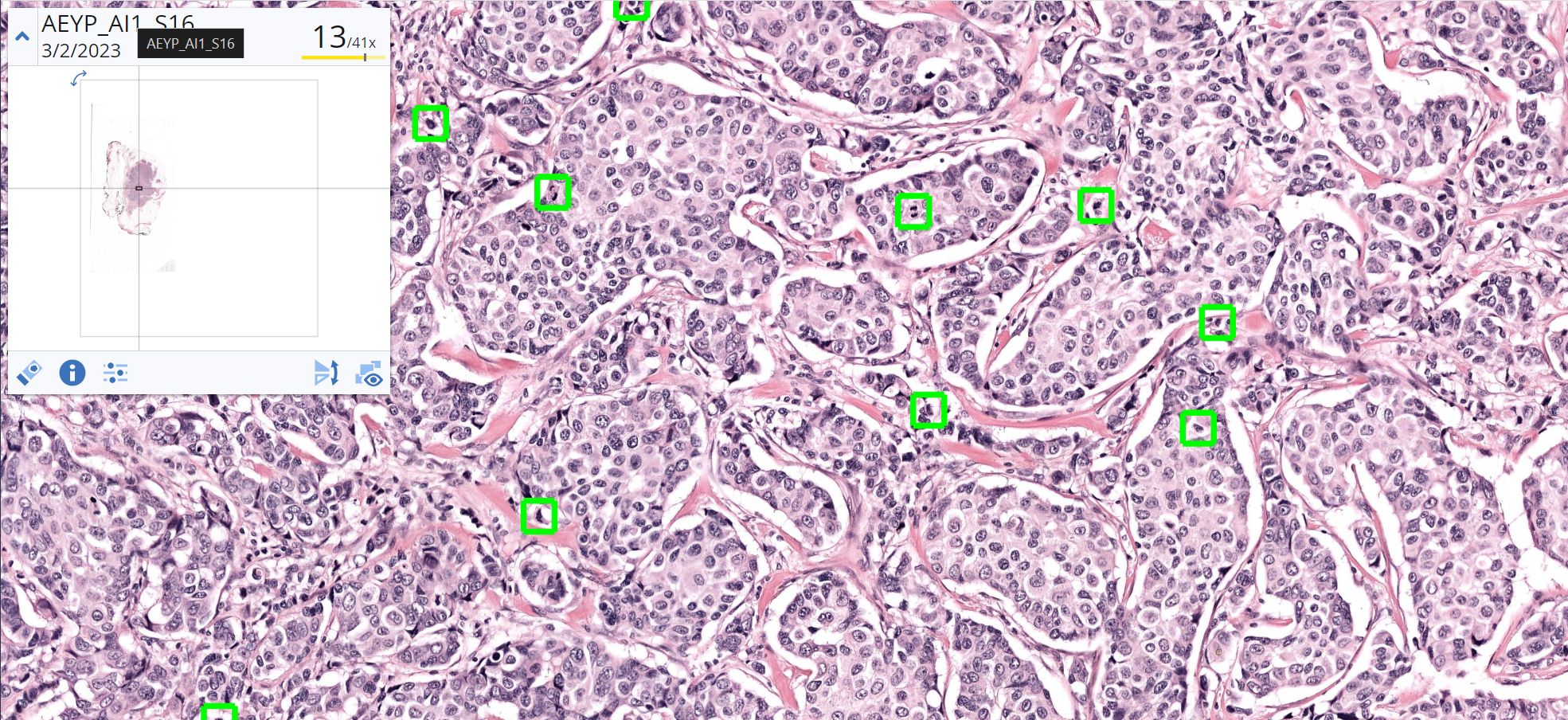
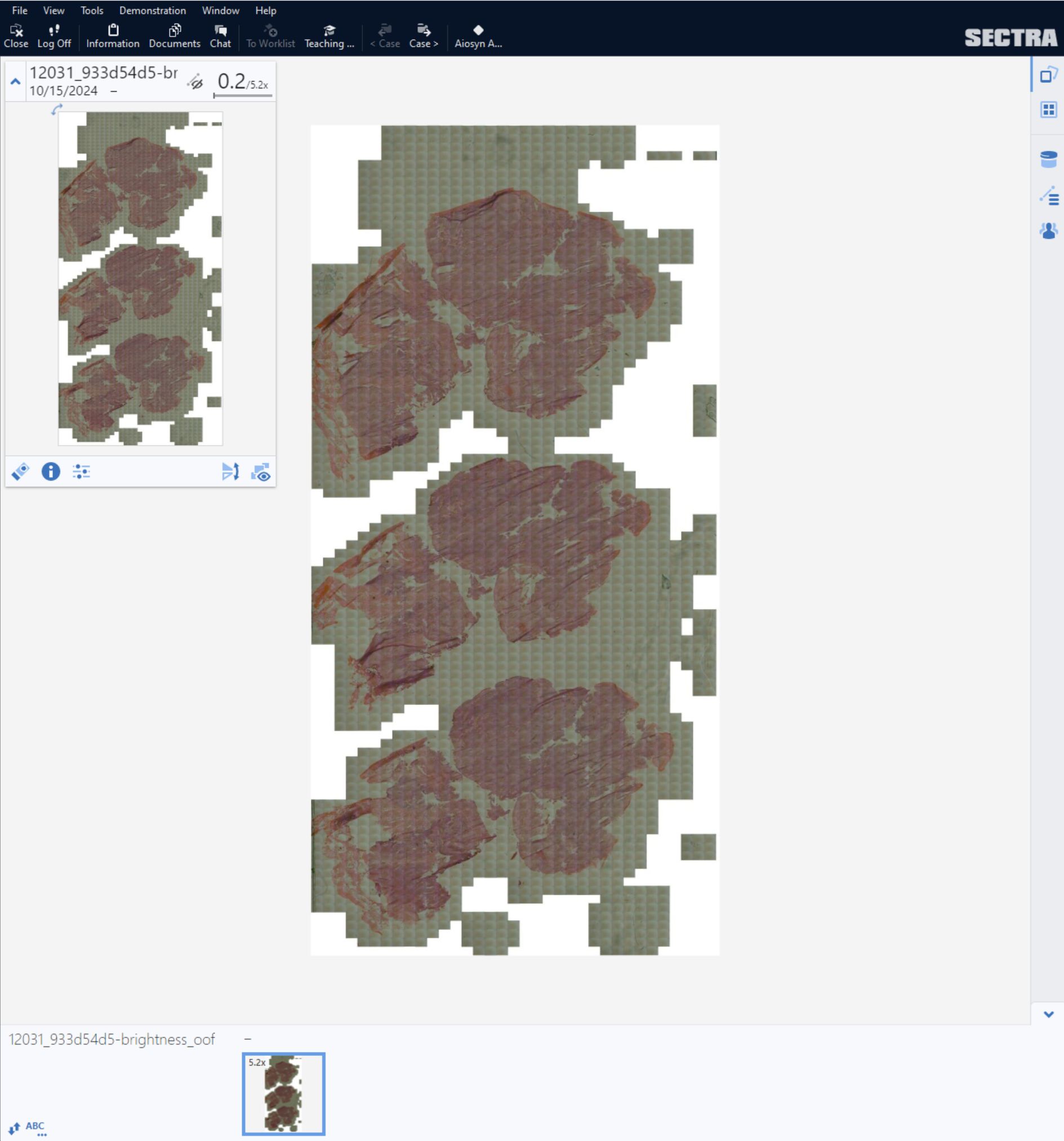
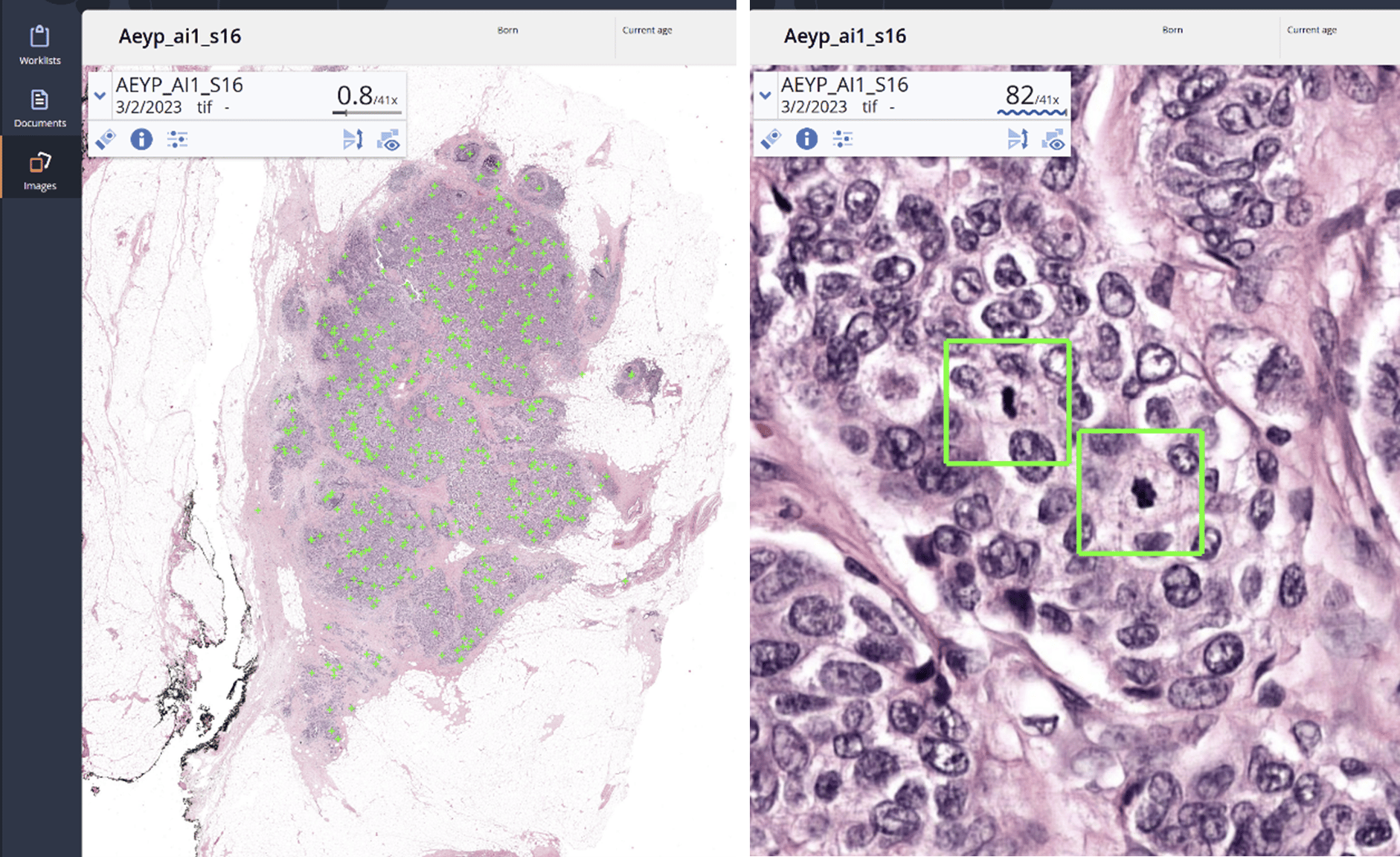
Nijmegen, the Netherlands – Aiosyn, a pioneering innovator in AI-powered pathology software for cancer and kidney disease, has introduced its external digital slide quality assessments to support laboratories in achieving good-quality histology slides—essential for accurate pathology diagnoses. Leveraging comprehensive, data-driven evaluations through AiosynQC Pulse, this service provides laboratories with insights to pinpoint common artifact issues, such as out-of-focus regions or tissue folds, improve digital slide quality, and comply with stringent quality requirements.
Patrick de Boer, CEO of Aiosyn

External quality assessments are vital for any laboratory quality system, but manual checks often fall short in addressing the demands of rising volumes and complex workflows. Aiosyn’s digital slide quality assessments use AI-powered artifact detection to deliver actionable key performance indicators (KPIs), helping labs improve pre-analytical processes, including technical setups and staff competencies. Through the AiosynQC Pulse platform, users can securely upload images without full integration, facilitating quick and efficient quality checks. By providing robust, traceable data, this service provides additional support for the accreditation efforts under ISO 15189 and ISO 17025 and fosters continuous improvement in digital pathology workflows.
Alongside external assessments that deliver performance snapshots at the preferred frequency, Aiosyn provides AiosynQC for laboratories seeking automated quality control within their daily workflow. This AI-powered solution flags poor WSIs before they reach the pathologist and allows for real-time alerts and continuous feedback, delivering actionable quality insights on an ongoing basis.
For more information on Aiosyn’s external digital slide quality assessments, please visit AiosynQC Pulse or contact Aiosyn at [email protected].
About Aiosyn
Based in the Netherlands, Aiosyn develops precision pathology software for kidney disease and breast cancer, integrating its solutions into standard pathology workflows. Aiosyn has been built upon more than 20 years of research experience in the field of pathology and is rooted in pathology practice.
More News
-
Aiosyn and Telemis advance breast cancer diagnosis with IVDR-certified AI technology
26 June, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn expands AI-powered quality control with incomplete scan detection
09 April, 2025 • By Anna Correas Grifoll
Read more -
Techcyte and Aiosyn collaborate to integrate AI-powered slide QC and mitotic counting into the Fusion™ digital pathology platform
20 March, 2025 • By Anna Correas Grifoll
Read more -
Techcyte, Pramana, and Aiosyn collaborate to advance digital pathology workflow with Edge AI Technology
20 March, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn and Pramana introduce real-time AI processing for kidney biopsy assessments
18 March, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn enhances its AI-powered quality control solution to detect white balance issues
05 February, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn’s kidney image analysis tools are now available on HistoWiz’s PathologyMap™
28 January, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn Mitosis Breast becomes the first AI-powered mitosis detection solution to achieve CE mark certification under IVDR
07 January, 2025 • By Anna Correas Grifoll
Read more