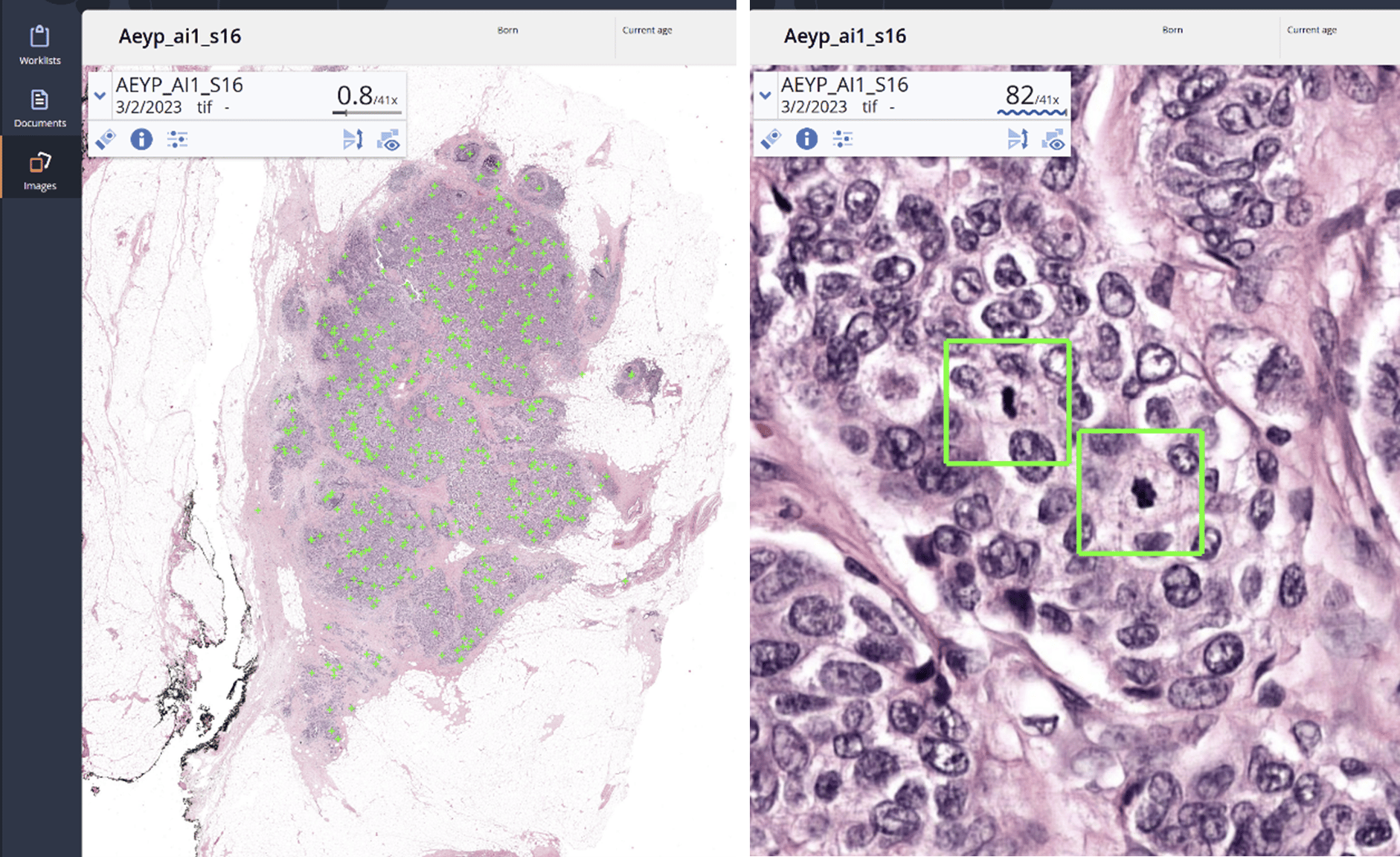
Nijmegen – Aiosyn, a medical software company that develops AI-powered pathology software, has achieved ISO:13485 certification, after successfully auditing its Quality Management System (QMS) by The British Standards Institution (BSI). Achieving this certification demonstrates Aiosyn’s commitment to producing high-quality AI-powered medical devices for pathology.

ISO 13485:2016 is a globally recognized standard for designing and manufacturing medical devices. The standard includes QMS requirements for design and development, document control, management, analysis, and continuous improvement. The certification demonstrates an organization’s ability to provide medical devices and related services that consistently meet the highest customer and applicable regulatory requirements.
“Achieving this ISO certification is a critical milestone for Aiosyn and the team. It demonstrates our progress in a fast-changing field of IVDR, AI, and digital pathology.”
Wouter Bulten, CIO of Aiosyn
Aiosyn aims to leverage this certification as an enabler of its regulatory strategy and product roadmap.
About Aiosyn
Aiosyn is a Dutch medical software company that develops AI-powered pathology solutions that will be integrated into standard pathology workflows. The Aiosyn teams have been built upon 20+ years of research experience in pathology and are rooted in the pathology practice.
For any inquiries about this update, please reach out to Wouter Bulten (CIO Aiosyn) through our contact form or at [email protected].
More News
-
Aiosyn and Telemis advance breast cancer diagnosis with IVDR-certified AI technology
26 June, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn expands AI-powered quality control with incomplete scan detection
09 April, 2025 • By Anna Correas Grifoll
Read more -
Techcyte and Aiosyn collaborate to integrate AI-powered slide QC and mitotic counting into the Fusion™ digital pathology platform
20 March, 2025 • By Anna Correas Grifoll
Read more -
Techcyte, Pramana, and Aiosyn collaborate to advance digital pathology workflow with Edge AI Technology
20 March, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn and Pramana introduce real-time AI processing for kidney biopsy assessments
18 March, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn enhances its AI-powered quality control solution to detect white balance issues
05 February, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn’s kidney image analysis tools are now available on HistoWiz’s PathologyMap™
28 January, 2025 • By Anna Correas Grifoll
Read more -
Aiosyn Mitosis Breast becomes the first AI-powered mitosis detection solution to achieve CE mark certification under IVDR
07 January, 2025 • By Anna Correas Grifoll
Read more